In the realm of healthcare, critical care devices are the cornerstone of life-saving interventions. These devices are indispensable in emergency rooms, intensive care units (ICUs), and other acute settings, ensuring patient survival and improving outcomes. The process of Critical Care Device Development requires meticulous planning, advanced engineering, and compliance with rigorous regulatory standards. As experts in medical and wellness device development, we’ll delve into the essentials of engineering critical care devices that prioritize patient success.
Table of Contents
Understanding Critical Care Device Development
Critical Care Device Development encompasses the design, prototyping, and manufacturing of devices used to monitor, diagnose, and treat patients in life-threatening conditions. These devices include ventilators, infusion pumps, patient monitoring systems, defibrillators, and dialysis machines. Given their role in critical care, they must be reliable, precise, and user-friendly for healthcare professionals.
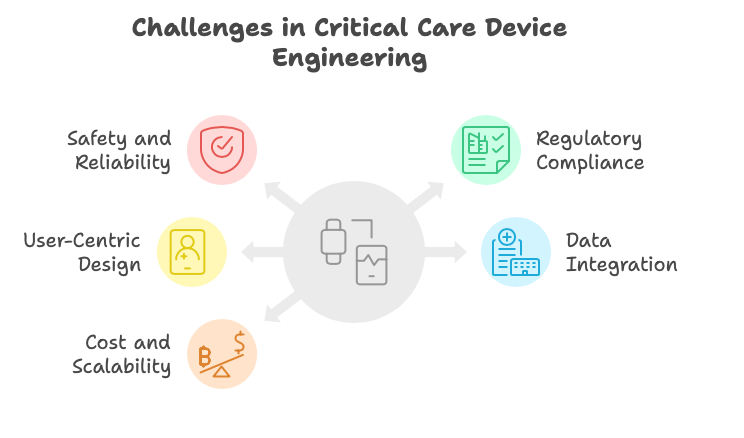
Key Challenges in Critical Care Device Engineering
Developing critical care devices presents unique challenges that demand innovative engineering solutions:
1. Safety and Reliability
Critical care devices must function without fail in high-stress environments. Redundancy in system design, robust software development, and thorough quality control processes are essential.
2. Regulatory Compliance
Compliance with standards such as FDA 21 CFR Part 820, ISO 13485, and IEC 60601-1 ensures safety and efficacy. Navigating these regulatory pathways requires expertise in documentation, testing, and audits.
3. User-Centric Design
Devices should be intuitive for medical professionals under pressure. Ergonomic design, clear interfaces, and minimized error risk are critical factors.
4. Data Integration and Connectivity
Modern critical care devices often need seamless integration with hospital systems and electronic health records (EHRs). Secure data transfer and interoperability are vital considerations.
5. Cost and Scalability
Balancing cutting-edge technology with cost-effectiveness is a constant challenge, especially when scaling for production and global distribution.
Steps in Critical Care Device Development
1. Needs Assessment
Understanding the unmet clinical needs is the first step. Collaborating with healthcare providers ensures the device addresses real-world challenges.
2. Conceptual Design
Early-stage brainstorming and prototyping help refine the product concept. This phase involves:
- Drafting initial designs.
- Simulating functionality using computer-aided design (CAD) software.
- Creating proof-of-concept prototypes.
3. Engineering and Prototyping
Here, the focus shifts to developing functional prototypes that can undergo preliminary testing. Engineering teams work on:
- Hardware design.
- Embedded software integration.
- Mechanical and electrical systems optimization.
4. Testing and Validation
Comprehensive testing ensures the device meets clinical and regulatory standards. This stage includes:
- Functional testing.
- Safety testing (e.g., electrical safety, electromagnetic compatibility).
- Usability testing with healthcare professionals.
5. Regulatory Submission
Submission to regulatory bodies like the FDA or EMA is a critical milestone. A complete technical file, clinical data, and risk analysis are part of the submission package.
6. Manufacturing and Scaling
Design for manufacturability (DFM) principles ensure the transition from prototype to mass production is smooth. Partnering with reliable manufacturers is essential to maintain quality.
7. Post-Market Surveillance
Even after launch, continuous monitoring is necessary to ensure device performance and safety. Gathering feedback helps improve future iterations.
Innovations Shaping Critical Care Device Development
Technological advancements are revolutionizing critical care devices:
Artificial Intelligence (AI) and Machine Learning
AI enhances diagnostics and predictive analytics, aiding in patient monitoring and treatment decisions.
Miniaturization
Smaller, portable devices enable critical care outside traditional hospital settings, like ambulances or home care environments.
Internet of Medical Things (IoMT)
IoMT connects critical care devices to cloud platforms for real-time monitoring and data analytics.
Advanced Materials
Biocompatible and durable materials ensure safety and longevity, even in demanding environments.
Success Stories in Critical Care Device Engineering
1. Portable Ventilators
In response to the COVID-19 pandemic, our team developed a compact ventilator the size of a laptop. It provided life-saving support in remote and resource-limited settings.
2. Smart Infusion Pumps
By incorporating AI, these pumps adjust medication dosage in real time, reducing human error and improving patient outcomes.
3. Wearable Monitoring Systems
These devices continuously monitor vital signs and alert clinicians to critical changes, allowing timely interventions.
Partnering for Excellence in Critical Care Device Development
Collaboration is key to creating impactful critical care devices. By partnering with a specialized Critical Care Device Development team, you can ensure:
- Compliance with stringent regulations.
- Seamless integration of cutting-edge technology.
- User-focused designs tailored for healthcare professionals.
Our expertise spans over 150 projects, ranging from smart crutches to innovative ventilators. With a proven track record in medtech innovation, we can help turn your vision into reality.
Conclusion
Engineering critical care devices is a high-stakes endeavor that demands precision, innovation, and an unwavering commitment to patient safety. By adhering to rigorous engineering principles, staying abreast of technological advancements, and prioritizing usability, these devices can truly revolutionize patient care.
If you’re exploring the development of a critical care device, partner with experts who understand the complexities and stakes involved. Together, we can create solutions that save lives and redefine healthcare.
Related Article:
Optical Medical Device Commercialization Guide
What specific challenge or innovation excites you most about critical care device development? Let’s discuss your ideas!



 430 Park Ave, New York, NY 10022, USA
430 Park Ave, New York, NY 10022, USA Paevalille tn 6, Office 84, Estonia, Tallinn, 13517
Paevalille tn 6, Office 84, Estonia, Tallinn, 13517 Barykadna St 7, Dnipro, Ukraine, 49000
Barykadna St 7, Dnipro, Ukraine, 49000