Developing a Minimum Viable Product (MVP) in the medical device industry is a critical step to transforming innovative ideas into tangible, market-ready solutions. MVP medical device development involves creating a functional prototype that captures the essential features of the device while meeting regulatory standards. This approach not only minimizes time and costs but also allows for early feedback, ensuring the final product addresses real-world clinical needs.
Table of Contents
This comprehensive guide explores the nuances of MVP medical device development, from ideation to prototype validation, emphasizing the strategies and tools that help medtech startups and innovators succeed.
Here’s a comprehensive spreadsheet-style knowledge guide packed with niche insights, actionable tips, and bold strategies for professionals in MVP medical device development and medtech innovation. Each row offers high-value advice based on deep expertise, addressing challenges and providing solutions you won’t find in surface-level content.
| Category | Insight/Tip | Actionable Strategy | Why It Matters |
|---|---|---|---|
| 1. Concept Validation | Start with a micro-prototype focused on one essential function. | Create a simplified working model using Arduino or Raspberry Pi to validate technical feasibility. | Saves time and costs while providing a tangible proof of concept to stakeholders. |
| 2. Regulatory Strategy | Don’t wait for the MVP to consider regulatory pathways. | Engage with a regulatory consultant to draft a pre-submission document for FDA or ISO compliance early. | Avoid costly redesigns and align the MVP with market requirements from the start. |
| 3. Stakeholder Feedback | Avoid generic user testing; target extreme users for feedback. | Identify power users or clinicians with advanced experience to stress-test usability and identify hidden flaws. | Extreme users push boundaries and uncover limitations that casual users might miss. |
| 4. Usability Testing | Simulate real-world failures during usability testing. | Add deliberate interruptions like power cuts or connectivity drops and observe user responses. | Real-world conditions ensure robustness and reliability, which are crucial in medtech. |
| 5. Material Selection | Choose materials not only for performance but also for manufacturability. | Work with manufacturers to validate that selected materials can scale without quality degradation. | A material that works in prototypes might fail in mass production due to variability or cost. |
| 6. Embedded Software | Prioritize modular coding practices to future-proof your device. | Write code with isolated functional modules that allow easy upgrades or feature additions. | Modularity reduces risk during updates and allows rapid iteration post-launch. |
| 7. Prototyping Tools | Skip generic CAD tools for medtech-specific design platforms. | Use platforms like SOLIDWORKS Premium with biocompatibility simulation plugins for detailed modeling. | Medtech-specific tools save time and ensure designs align with industry standards. |
| 8. Supply Chain Risks | Vet secondary suppliers to avoid single points of failure. | Maintain a list of backup suppliers and pre-approve their materials to mitigate risks of primary supplier disruption. | Reduces delays caused by unforeseen supply chain issues, especially for critical components. |
| 9. Clinical Insights | Collect early feedback from nurses, not just doctors. | Nurses often identify practical usability issues that improve design for all users. | Ensures that the device is truly user-friendly in everyday clinical environments. |
| 10. Battery Optimization | Design power systems with a minimum of 2x redundancy for critical devices. | Integrate both primary and backup battery systems, testing hot-swapping capabilities. | Avoids failures in life-critical applications, a key differentiator for medtech devices. |
| 11. AI/ML Integration | Train algorithms on diverse datasets, including edge cases. | Partner with hospitals to access anonymized real-world data and integrate rare but critical scenarios into training sets. | Increases algorithm robustness and regulatory compliance for safety-critical AI components. |
| 12. Data Security | Implement security at the firmware level, not just the software level. | Use hardware-level encryption (e.g., Trusted Platform Modules) to safeguard patient data from the ground up. | Protects against sophisticated cybersecurity threats targeting embedded systems. |
| 13. Quality Assurance | Create a real-time QA dashboard to monitor device performance metrics during testing. | Implement IoT-connected sensors in prototypes to log data continuously to a centralized system for analysis. | Speeds up debugging and provides actionable insights to improve quality. |
| 14. Manufacturing Scalability | Design assembly lines with flexibility for small-batch production during early commercialization. | Choose manufacturers with agile capabilities who can scale batches up or down without high switching costs. | Ensures smooth scaling from MVP to full commercialization while controlling costs. |
| 15. Investor Pitches | Avoid generic ROI claims; showcase real user stories from MVP tests. | Record interviews with clinicians or patients who tested the MVP and share their feedback during investor presentations. | Creates a strong emotional connection and credibility, differentiating your pitch from competitors. |
| 16. Environmental Factors | Test the MVP in extreme environmental conditions (humidity, heat, etc.). | Use environmental chambers to test performance and durability under challenging conditions. | Ensures the device remains reliable in diverse deployment scenarios, like tropical regions or high-altitude clinics. |
| 17. IP Strategy | File provisional patents for individual components during MVP development. | Break down your device into subsystems and patent each innovation separately to build a strong IP portfolio. | Strengthens market position and increases valuation for potential investors or acquirers. |
| 18. User Documentation | Develop intuitive, visual-first user guides during the MVP stage. | Use infographics and videos instead of text-heavy manuals to simplify onboarding for end users. | Reduces learning curve and improves adoption rates among clinicians and patients. |
| 19. Device Connectivity | Design for offline functionality in IoT devices. | Include offline data logging and synchronization features for devices used in low-connectivity areas. | Expands usability across remote or underserved regions, increasing market potential. |
| 20. Competitive Edge | Study regulatory delays faced by competitors and plan proactively. | Analyze public FDA submissions to learn common pitfalls and implement safeguards to avoid similar issues. | Positions your product to achieve faster approval and a first-mover advantage in the market. |
What is MVP Medical Device Development?
An MVP (Minimum Viable Product) in the medtech sector is a functional version of a medical device that includes the core features necessary to solve a specific clinical problem. Unlike a fully realized product, an MVP is developed with minimal resources to validate the concept and gather feedback from stakeholders like clinicians, regulators, and potential users.
The primary goals of MVP medical device development are to:
- Demonstrate the device’s feasibility and functionality.
- Minimize development risks and costs.
- Accelerate time to market.
- Incorporate user and regulatory feedback into subsequent iterations.
Steps in MVP Medical Device Development
1. Define the Problem and Solution
The process begins with a deep understanding of the clinical problem your device aims to solve. This involves:
- Identifying the unmet need in the healthcare sector.
- Researching existing solutions and gaps in the market.
- Consulting with clinicians, patients, and stakeholders to validate the concept.
Example:
A wearable ECG monitor might address the need for continuous cardiac monitoring in outpatients. Understanding this specific need defines the core functionality required for the MVP.
2. Establish Requirements and Features
Clearly outline the essential features your MVP must include. These should focus on solving the primary clinical problem without unnecessary complexity.
- Functional Requirements: What the device should do (e.g., monitor, measure, or assist).
- Performance Metrics: Define measurable outcomes (e.g., accuracy, reliability).
- User Experience: Prioritize usability for patients and clinicians.
Pro Tip:
Limit the MVP to “must-have” features to reduce complexity, cost, and development time.
3. Develop a Prototype
The prototype is the tangible realization of your MVP concept. Depending on your resources, this stage might include:
- Low-Fidelity Prototypes: Initial models using simple materials to validate design concepts.
- High-Fidelity Prototypes: Functional prototypes with working components for testing core functionality.
Tools:
- CAD software for design.
- 3D printing for rapid prototyping.
- Simulation tools for testing functionality in virtual environments.
4. Ensure Regulatory and Compliance Alignment
Medical devices are subject to stringent regulations, even at the MVP stage. Key steps include:
- Identifying applicable regulations (e.g., FDA Class I, II, or III).
- Conducting risk analysis according to ISO 14971.
- Documenting design controls and testing protocols.
Example:
For a wearable glucose monitor, compliance with biocompatibility standards (ISO 10993) and electromagnetic compatibility (EMC) testing are critical even in the MVP phase.
5. Conduct Testing and Validation
Testing is a critical phase of MVP development to ensure safety, reliability, and effectiveness. Key tests include:
- Functional Testing: Verify that the device performs its intended function.
- Usability Testing: Ensure the design is intuitive for end users.
- Preclinical Studies: Simulate clinical conditions to validate performance.
Example:
A portable ultrasound device’s MVP might undergo testing to validate image clarity and power efficiency under varied use cases.
6. Gather Feedback and Iterate
The MVP is not a finished product—it’s a stepping stone. Use it to gather feedback from:
- Clinicians and End Users: Test usability, reliability, and relevance.
- Regulators: Identify potential compliance gaps early.
- Investors: Demonstrate the product’s viability and attract funding.
Action Plan:
- Host user workshops or pilot studies.
- Use feedback to prioritize changes for the next iteration.
7. Prepare for Full-Scale Development
Once the MVP has validated the concept and undergone refinements, transition to full-scale development. Key focuses include:
- Refining the design for manufacturability.
- Scaling up production.
- Preparing for clinical trials and regulatory submissions.
Pro Tip:
Leverage MVP insights to streamline the transition to full product development, reducing time-to-market.
Challenges in MVP Medical Device Development
1. Balancing Speed with Compliance
Rushing to build an MVP may lead to overlooking critical regulatory requirements. Engaging regulatory consultants early can mitigate this risk.
2. Cost Management
Developing even a simple prototype can be expensive. Prioritize cost-effective tools and materials without compromising functionality.
3. Cross-Disciplinary Coordination
MVP development requires collaboration between engineers, designers, clinicians, and regulatory experts. Misalignment can result in delays and increased costs.
Case Studies in MVP Medical Device Development
2. Portable Ventilator During the COVID-19 pandemic, a medtech company applied MVP Medical Device Development principles to rapidly produce a ventilator with core functionalities to address shortages. Feedback from clinicians informed rapid iterations, enabling regulatory approval within months. This case highlights how MVP Medical Device Development can accelerate time-to-market for critical healthcare solutions.
1. Wearable Glucose Monitor
A startup leveraged MVP Medical Device Development methodology to create a streamlined glucose monitor to continuously track blood sugar levels. By focusing on essential features—accuracy and connectivity—the team successfully demonstrated feasibility and secured investor funding for further development. This approach to MVP Medical Device Development allowed them to validate their core technology before adding more complex features
3. AI-Powered Diagnostic Tool An MVP Medical Device Development team created a simplified AI diagnostic tool focused solely on analyzing lung X-rays. This targeted approach allowed them to validate their algorithm’s accuracy before expanding to other diagnostic capabilities, reducing development time by 60%.
4. Remote Patient Monitoring System Using MVP Medical Device Development methodology, a healthcare technology company built a streamlined monitoring system that tracked only vital signs and medication adherence. This focused solution enabled real-world testing in small clinics, uncovering critical integration challenges that would have been costly to address later.
5. Minimally Invasive Surgical Device A surgical equipment manufacturer applied MVP Medical Device Developmentprinciples to create a prototype surgical tool with only essential functionalities. By limiting scope to core cutting and sealing capabilities, they secured early surgeon feedback and validation before investing in advanced features like tissue recognition.
6. Digital Therapeutic Application Following MVP Medical Device Development best practices, a digital health startup developed a simplified behavioral therapy application focused on a single mental health condition. This strategic limitation allowed them to establish clinical efficacy through focused trials before expanding their therapeutic offerings.
Best Practices for MVP Medical Device Development
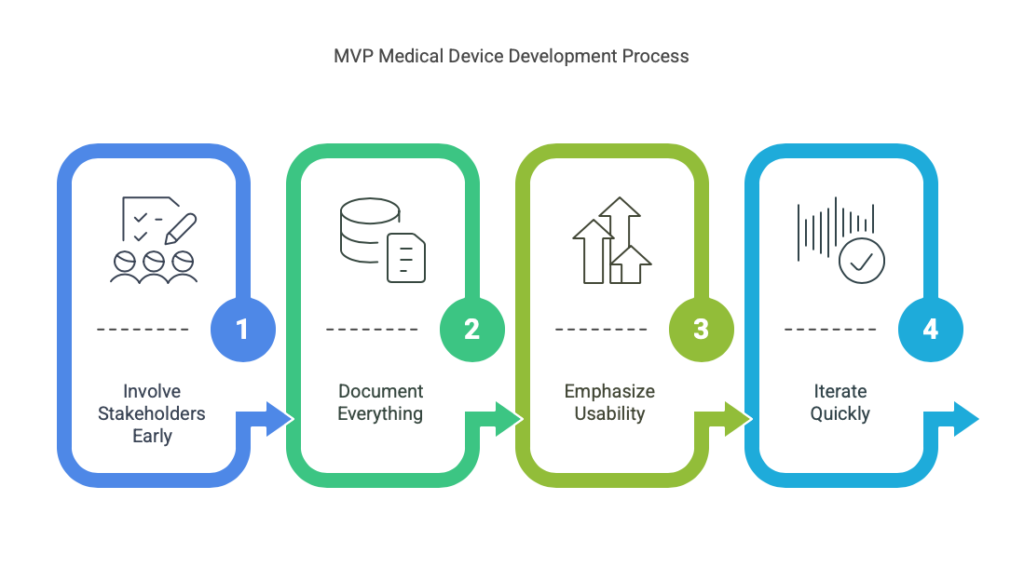
- Involve Stakeholders Early: Engage end users, regulators, and investors during the MVP stage to ensure alignment with market and compliance needs.
- Document Everything: Maintain detailed records of testing, design iterations, and feedback for future regulatory submissions.
- Emphasize Usability: Prioritize designs that are intuitive and user-friendly, as this significantly impacts adoption rates.
- Iterate Quickly: Use rapid prototyping tools to refine the MVP based on continuous feedback.
Conclusion
MVP medical device development is a strategic approach that accelerates innovation while managing risks and costs. By focusing on essential features, aligning with regulatory requirements, and gathering early feedback, medtech innovators can bring impactful devices to market faster. The process transforms ideas into reality, ensuring devices address real-world clinical needs and are poised for commercial success.
At OVA Solutions, we specialize in guiding medtech companies through the MVP development process. With expertise in prototyping, regulatory compliance, and hardware engineering, we help innovators create functional, compliant, and market-ready medical devices. Whether you’re building wearable health monitors, diagnostic tools, or surgical equipment, we’re here to bring your vision to life.
Ready to start your MVP journey? Contact OVA Solutions today and turn your idea into reality!


