In the medical device industry, regulatory compliance is a cornerstone of ensuring safety, efficacy, and market readiness. It represents the rigorous standards and processes that govern the design, development, manufacturing, and distribution of medical devices. For companies aiming to bring innovative devices to market, understanding and adhering to regulatory compliance requirements is non-negotiable. This comprehensive guide explores the essentials of regulatory compliance, its significance, key regulations, and actionable strategies for success in the medical and wellness device industry.
Table of Contents
What is Regulatory Compliance in Medical Devices?
Regulatory compliance refers to the adherence to laws, guidelines, and standards set by regulatory bodies to ensure that medical devices are safe, effective, and fit for their intended use. This involves meeting requirements for:
- Design and development processes.
- Risk management and usability testing.
- Manufacturing quality systems.
- Pre-market approvals or clearances.
- Post-market surveillance.
Importance of Regulatory Compliance
- Patient Safety: Protects users by ensuring devices meet stringent safety and performance criteria.
- Market Access: Compliance is mandatory for entering global markets like the U.S., Europe, and Asia.
- Brand Reputation: Builds trust with stakeholders by demonstrating commitment to quality.
- Risk Mitigation: Reduces the likelihood of recalls, penalties, and legal liabilities.
- Innovation Facilitation: Provides a structured framework for developing groundbreaking technologies.
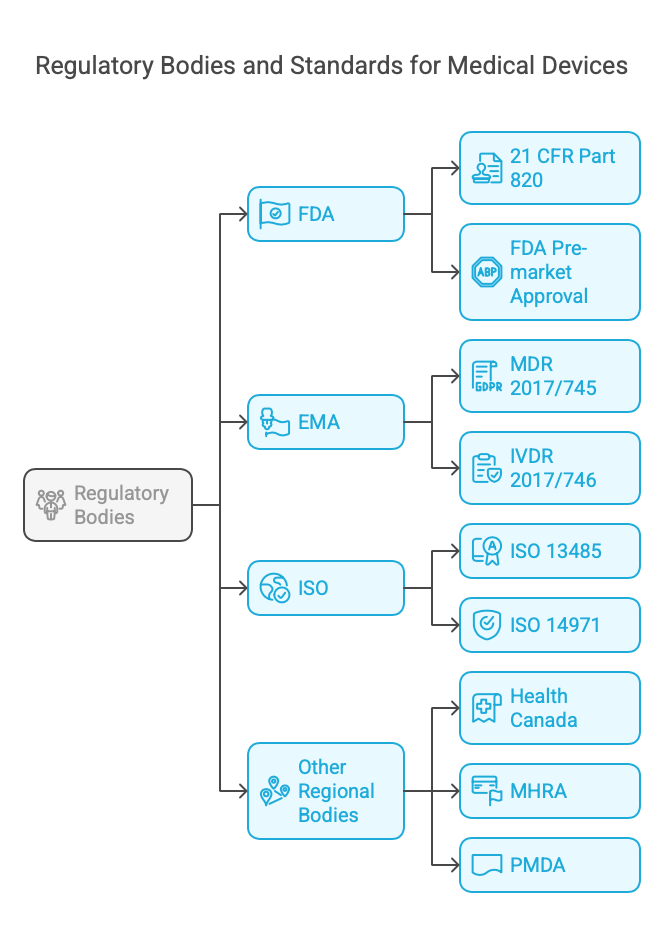
Key Regulatory Bodies and Standards
1. U.S. Food and Drug Administration (FDA)
- Scope: Regulates medical devices in the United States.
- Key Standards:
- 21 CFR Part 820: Quality System Regulation (QSR).
- FDA Pre-market Approval (PMA) and 510(k) clearances.
2. European Medicines Agency (EMA)
- Scope: Oversees medical device regulations in the European Union.
- Key Standards:
- Medical Device Regulation (MDR) 2017/745.
- In Vitro Diagnostic Regulation (IVDR) 2017/746.
3. International Organization for Standardization (ISO)
- Scope: Provides global standards for medical devices.
- Key Standards:
- ISO 13485: Quality Management Systems.
- ISO 14971: Risk Management for Medical Devices.
4. Other Regional Bodies
- Health Canada.
- Medicines and Healthcare products Regulatory Agency (MHRA) in the UK.
- Japan’s Pharmaceuticals and Medical Devices Agency (PMDA).
Steps to Achieve Regulatory Compliance
1. Understand Regulatory Requirements
- Identify applicable regulations based on the target market and device classification.
- Engage with regulatory experts to clarify complex requirements.
2. Implement a Quality Management System (QMS)
- Use ISO 13485 or an equivalent standard as the foundation for your QMS.
- Document all processes, from design to post-market activities.
3. Conduct Risk Management
- Perform risk assessments using tools like Failure Mode and Effects Analysis (FMEA).
- Develop and implement mitigation strategies for identified risks.
4. Perform Design and Development Validation
- Validate that the device meets user needs and intended purposes.
- Conduct usability testing to ensure safe and effective operation.
5. Compile Technical Documentation
- Prepare a comprehensive technical file, including design history, risk analyses, and clinical evaluations.
- Submit this documentation to regulatory authorities for approval.
6. Obtain Necessary Certifications
- Secure certifications like CE marking for the EU or FDA clearance for the U.S.
- Ensure that labels and instructions meet regulatory standards.
7. Maintain Post-Market Surveillance
- Monitor device performance and collect user feedback.
- Report adverse events and implement corrective actions as needed.
Common Challenges in Regulatory Compliance
1. Complex and Evolving Regulations
- Challenge: Keeping up with changing regulatory landscapes.
- Solution: Stay informed through regulatory updates and engage with compliance experts.
2. Resource Intensiveness
- Challenge: High costs and time requirements for compliance activities.
- Solution: Use digital tools and software to streamline processes.
3. Global Variability
- Challenge: Different regulations across markets.
- Solution: Develop a global regulatory strategy and adapt documentation accordingly.
4. Data Management
- Challenge: Managing vast amounts of documentation and data.
- Solution: Implement robust document control systems.
Tips for Navigating Regulatory Compliance
1. Engage Early with Regulatory Bodies
- Seek pre-submission meetings with authorities to clarify requirements and expectations.
2. Leverage Digital Tools
- Use regulatory compliance software to manage documentation, audits, and submissions.
3. Invest in Training
- Train staff on regulatory standards and compliance processes.
- Keep teams updated on regulatory changes.
4. Plan for Post-Market Surveillance
- Develop a system for tracking device performance and addressing issues proactively.
5. Collaborate with Experts
- Partner with consultants and regulatory specialists to navigate complex requirements.
Benefits of Regulatory Compliance
- Enhanced Patient Safety: Ensures devices are safe and effective for use.
- Market Expansion: Facilitates entry into global markets.
- Operational Excellence: Encourages robust internal processes.
- Brand Credibility: Builds trust with healthcare providers and patients.
- Reduced Legal Risks: Minimizes exposure to lawsuits and penalties.
Future Trends in Regulatory Compliance
1. AI and Machine Learning
- Regulatory frameworks for AI-driven devices are evolving to address unique risks and opportunities.
2. Digital Compliance Tools
- Adoption of cloud-based platforms for real-time monitoring and compliance tracking.
3. Global Harmonization
- Efforts to align regulations across regions to simplify global compliance.
4. Focus on Cybersecurity
- Increased emphasis on securing connected devices against cyber threats.
Conclusion
Regulatory compliance is an integral part of bringing safe, effective, and innovative medical devices to market. By understanding the requirements, implementing robust systems, and addressing challenges proactively, manufacturers can ensure market readiness and long-term success. As the regulatory landscape continues to evolve, staying agile and informed will be key to navigating compliance efficiently.
For more insights into medical device engineering and market readiness, explore our comprehensive guide to optical medical device commercialization.



 430 Park Ave, New York, NY 10022, USA
430 Park Ave, New York, NY 10022, USA Paevalille tn 6, Office 84, Estonia, Tallinn, 13517
Paevalille tn 6, Office 84, Estonia, Tallinn, 13517 Barykadna St 7, Dnipro, Ukraine, 49000
Barykadna St 7, Dnipro, Ukraine, 49000